Technology Transfer Information
Technology Transfer Information
Introduction
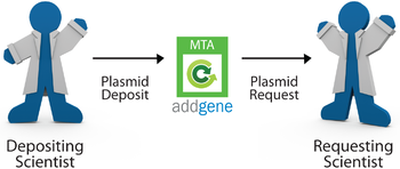
Addgene is a global, nonprofit organization dedicated to making it easier for scientists to share plasmids. As a repository, institutions provide (or deposit) materials with us for distribution. Then, institutions order (or request) materials from us. Addgene distributes most materials under the terms of the UBMTA. All Addgene orders require the acceptance of terms of use for the requested items.
- As part of each deposit we require a Deposit and Distribution Agreement.
- For most requests we are required to facilitate a Material Transfer Agreement (MTA).
- We have developed streamlined, user-friendly, and efficient systems for both depositing and requesting.
- We provide peace of mind that each transfer is accompanied by the proper legal paperwork for each transfer and provide detailed reporting to ensure that your institution is aware of both outgoing and incoming materials.
Contact Us

Questions about your Order: [email protected]
General Questions/Comments: [email protected]
Deposit Process
Deposit and Distribution Agreement Process
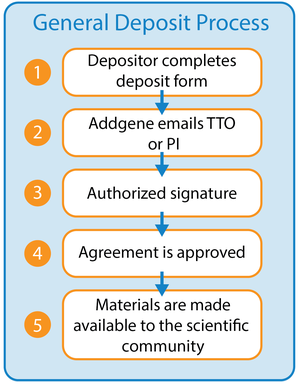
After a scientist initiates a plasmid deposit to Addgene, Addgene will generate a Deposit and Distribution Agreement detailing the materials in the deposit. Visit our Deposit page for more information about depositing plasmids.
The Deposit and Distribution Agreement is sent to the authorized person/office who approves deposit agreements, or to the depositing Principal Investigator (PI) to obtain approval from the authorized person/office. Depending on your institution's deposit agreement processing requirements, Addgene's Deposit and Distribution Agreements can either be approved through a signature on PDF, electronic signature, or electronic approval through your institution's Addgene Technology Transfer Account.
To read more about how to approve materials through a Technology Transfer Account, please find more information regarding our Master Deposit Agreements below.
Deposit Agreement Options
Addgene deposits can be approved in one of two ways: a One-Time Deposit and Distribution Agreement or a Master Deposit Agreement.
One-Time Deposit and Distribution Agreement
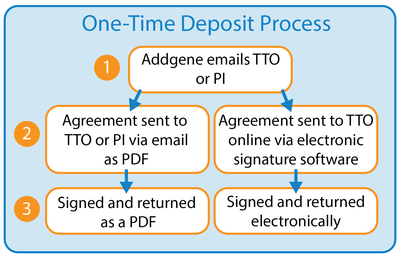
- A One-Time Agreement can be signed by an authorized person either through a PDF/paper signature or through electronic signature. After the deposit is initiated by the researcher, the One-Time Deposit Agreement is generated (1).
- This agreement is sent to either the PI or the Providing Institution’s Technology Transfer Office (or Office of Sponsored Programs, Legal Office, etc.) (2).
- Agreement Approval (3)
- PDF Option - If the agreement is sent to the PI, Addgene will send this agreement via email as a PDF. This agreement can then be signed by an authorized person and returned via email/PDF.
- Electronic Option - If the agreement is sent to the Providing Institution’s Technology Transfer Office (TTO), the agreement will be sent either via email as a PDF, or it will be sent for electronic signature online. These two options will differ depending on the TTO’s preference.
Master Deposit Agreement
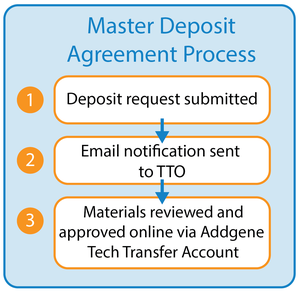
- With a Master Deposit Agreement your institution can sign the terms upfront once for all future deposits (1).
- Then, the materials themselves can be reviewed and approved through an Addgene Technology Transfer Account that Addgene will create for the authorized office (2). This office can be a Technology Transfer Office, Office of Sponsored Programs, Office of Technology Development, Legal Office or similar.
- After the Master Deposit Agreement is signed, the authorized office will receive email notifications each time there is a new deposit (3). We must have a centralized office where all deposit notifications can be sent for this Master Deposit Agreement option.
- Over 150 institutions have currently signed this agreement and are using this process.
If your institution requires additional information (through an institutional form or similar) from the PI or lab before outgoing materials can be approved, we have the ability to host a Questionnaire for your institution which would incorporate your internal questions into our deposit processes.
To view sample One-Time and Master Deposit Agreements, please see our Sample Agreements in our Resources section.
Request Process for Plasmids
Material Transfer Agreement Process
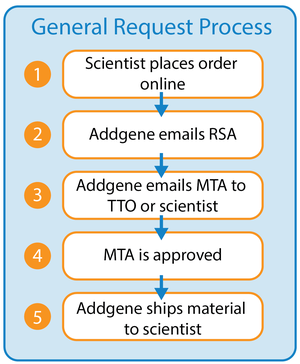
After a scientist initiates a plasmid request from Addgene, we will generate a Recipient Scientist Acknowledgement that will ask the scientist to confirm that they understand the terms of the MTA. More information about requesting plasmids from Addgene can be found in the ordering section of our Help Center.
We can also include institutional forms during this ordering process so that scientists can submit additional information to your office at the same time they acknowledge the MTA. We can do so by linking to your institution’s website, or by hosting a Questionnaire through our website. Once the scientist completes those steps, Addgene will generate their request’s MTA and send it out for approval via one of the options below. Once we receive the signed MTA, we will prepare the order for shipment.
Material Transfer Agreement Options
There are multiple options for approving an MTA, some of which are more efficient for scientists and technology transfer officers alike. These options are the Paper Method, Electronic Approval Method (eMTA), and the Auto Approval Method.
Paper Method
Average Approval Time: ∼4 days
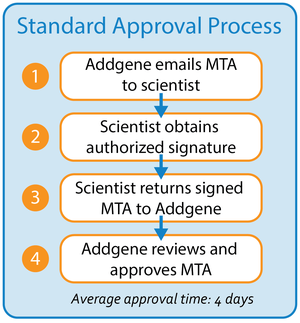
This is the default option through which the MTA is sent to the requesting scientist (1), who must then print the MTA and forward it to the appropriate signatory’s office for approval.
- Scientist must identify the authorized signatory and go to them for signature (2). Read more about who is authorized to sign MTAs.
- Submitting an MTA with an unauthorized signature will delay your order by at least 1 business day.
- The signed MTA can be submitted to Addgene (3) by uploading the PDF file to the Order Status page, sending the PDF file via email, or sending the packet fax.
- Addgene then reviews and approves the MTA once we receive it (4), which will then allow us to prepare the requested materials for shipment.
Electronic Approval Method (eMTA)
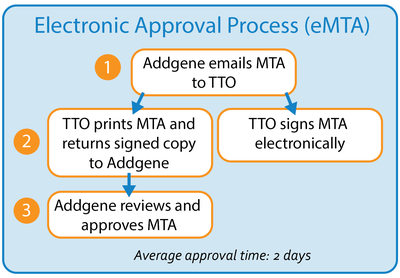
Average Approval Time: ∼2 days
Rather than the scientist handling each MTA, this option allows all agreements for an institution to be sent directly to the office authorized to approve them.
- Addgene keeps email addresses on file for the authorized office, which we use to notify the office about each pending MTA (1).
- The scientist is also provided with those email addresses in case they have any questions about their institution's approval process. Otherwise, they do not need to be involved in the MTA process, allowing them to continue focusing on their research.
- The authorized office can then approve each MTA electronically through our website (2), a feature that is not available through the Paper Method described above.
- As soon as the electronic signature for every implementing letter in the MTA is submitted, the requested materials will be prepared for shipment. Addgene does not need to review the signed agreement because we know it went directly to the authorized office (3).
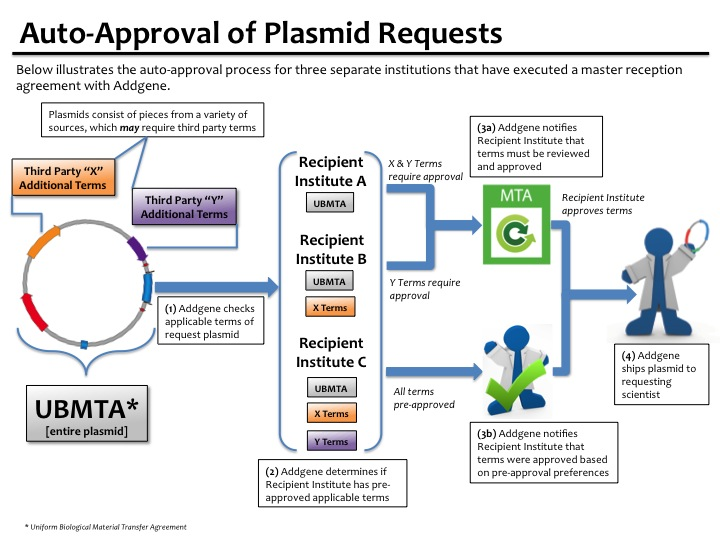
Auto Approval Method
Average Approval Time: Mostly Automatic
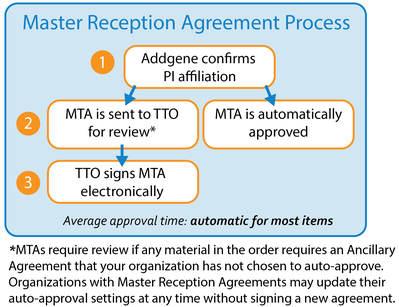
This option is only possible once your institution signs a master reception agreement with Addgene.
- Once a Master Reception Agreement is negotiated and signed by the authorized office, they can then choose to have MTAs auto-approved rather than submitting electronic signatures each time.
- With this option, after a scientist acknowledges the MTA through our website (as well as any institution forms when applicable), Addgene will then verify that the scientist’s PI is truly affiliated with the requesting institution (1).
- As soon as this verification is completed, the MTA will either be automatically approved (2), or sent to the authorized office for review depending on the settings they choose for their institution (2,3).
- If we cannot verify the PI’s affiliation, we will contact the PI and/or the requesting scientist directly.
- Auto-approval allows us to begin preparing the requested materials for shipment as soon as the PI affiliation verification is completed, rather than waiting for the MTA to be reviewed.
To view a sample Implementing Letter and Master Reception Agreement, please see our Sample Agreements in our Resources section.
Technology Transfer Accounts
An Addgene Technology Transfer Account, or Tech Transfer Account, provides your institution with the ability to approve and track incoming and outgoing materials. You can sign up for a Tech Transfer Account by emailing [email protected].
Benefits of a Tech Transfer Account
-
Easy reagent tracking
Addgene's intuitive online Tech Transfer Account pages allow you to view an inventory of deposited and requested materials anytime, and give you access to digital copies of executed MTAs.
Addgene's Tech Transfer Account sends you an email summary of material transfers through Addgene on a daily, weekly, or monthly basis. Set your preferences through your Tech Transfer Account page or email [email protected] for help.
-
Easy approval of plasmid deposits and requests
If your institution is on a Master Agreement for either Requests or Deposits, you can approve incoming or outgoing materials, directly through your Tech Transfer Account, thereby saving your office time and streamlining the Material Transfer process.
Resources
Questionnaires

Does your institution’s Technology Transfer Office require researchers to complete an internal form or questionnaire before processing deposit agreements or MTAs?
Addgene can help by hosting these questions on your institution’s behalf. Read our guide for more information:
Questionnaire Guide 1.6 MBSample Agreements

Browse samples of the agreements used in material requests and deposits:
Third Party Information

Many of Addgene’s plasmids contain technologies that are owned and/or licensed by third parties. For these plasmids, an ancillary agreement or limited use label license may be involved.
For more information about ancillary agreements or limited use label licenses, please visit our Terms Accounting for Third Party Rights page.
Technology Transfer Newsletter

To read our newsletters, please visit our Technology Transfer Newsletters page.
Information for Your Scientists

Do researchers at your institution receive many plasmid requests for their materials from their colleagues?
- If so, encourage them to deposit their materials with Addgene!
- When a researcher deposits with Addgene, the depositing institution and researcher benefit from the following:
- MTA compliance and tracking.
- Satisfy grant sharing requirements.
- Depositing is easy and free.
- Labs save time and money associated with shipping samples. Plus, earn free Addgene plasmids.
- All plasmids and data are securely archived for the future.
- Improved visibility for research as the scientist joins a global repository with 5,758 depositing labs.
If you would like to reach out to a researcher who you think could benefit from depositing, share information about Addgene with them:
Through press releases, update emails, or newsletters
Modify this template to suit your institution's needs. Log into your Tech Transfer Account to find and add information and stats specific to your institution.
Read more about the benefits of a Tech Transfer Account here.
Addgene is a nonprofit plasmid repository that facilitates research by authenticating, archiving, and distributing plasmids and their associated data. Addgene has worked with 5,758 laboratories from around the world to assemble a collection of 134,919 plasmids. The plasmid collection represents a broad range of disciplines and can be used for a variety of functions such as genome engineering, gene knockdown, viral gene delivery, as well as many applications for use in a wide range of cells and organisms.
Addgene is continuing to expand its collection and invites you to join the community. Do you receive requests for plasmids you've made in your lab? You can deposit them to Addgene at no cost. You will have more time for your research, Addgene will handle the samples and MTA compliance, and you will have a full record of where your plasmids have been shipped.
To learn more, visit www.addgene.org.
If one of your researchers is interested in depositing, have them visit our Deposit Page.
Video: MTA Request Guide
Watch our video below for information about:
- What an MTA is
- How you get an MTA started
- Approval of an MTA
Click the link to open and close the video window. If you have trouble accessing the video, please try viewing on YouTube.
Video: MTA Signature Guide
Watch our video below for information about:
- Who is authorized to sign MTAs
- MTA submission details
- Whether you can reuse MTAs for orders
Click the link to open and close the video window. If you have trouble accessing the video, please try viewing on YouTube.